

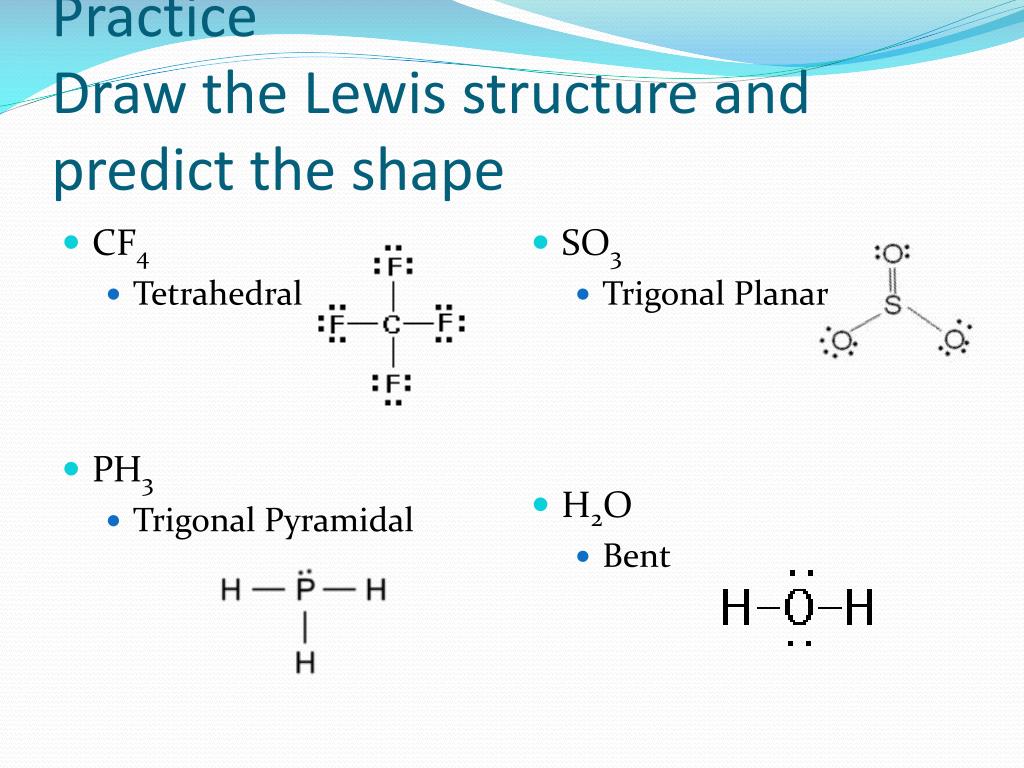
The electron-pair geometries will be the same as the molecular structures when there are no lone electron pairs around the central atom, but they will be different when there are lone pairs present on the central atom.įor example, the methane molecule, CH 4, which is the major component of natural gas, has four bonding pairs of electrons around the central carbon atom the electron-pair geometry is tetrahedral, as is the molecular structure ( ). The structure that includes only the placement of the atoms in the molecule is called the molecular structure. We differentiate between these two situations by naming the geometry that includes all electron pairs the electron-pair geometry. Molecular structure describes the location of the atoms, not the electrons. The electron-pair geometries shown in describe all regions where electrons are located, bonds as well as lone pairs. It is important to note that electron-pair geometry around a central atom is not the same thing as its molecular structure. The bond angle is 180° ( ).Įlectron-pair Geometry versus Molecular Structure With two bonds and no lone pairs of electrons on the central atom, the bonds are as far apart as possible, and the electrostatic repulsion between these regions of high electron density is reduced to a minimum when they are on opposite sides of the central atom. The Lewis structure of BeF 2 ( ) shows only two electron pairs around the central beryllium atom. Other interactions, such as nuclear-nuclear repulsions and nuclear-electron attractions, are also involved in the final arrangement that atoms adopt in a particular molecular structure.Īs a simple example of VSEPR theory, let us predict the structure of a gaseous BeF 2 molecule. We should understand, however, that the theory only considers electron-pair repulsions. VSEPR theory predicts the arrangement of electron pairs around each central atom and, usually, the correct arrangement of atoms in a molecule. The electrostatic repulsion of these electrons is reduced when the various regions of high electron density assume positions as far from each other as possible.

The electrons in the valence shell of a central atom form either bonding pairs of electrons, located primarily between bonded atoms, or lone pairs. The VSEPR model assumes that electron pairs in the valence shell of a central atom will adopt an arrangement that minimizes repulsions between these electron pairs by maximizing the distance between them. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.Valence shell electron-pair repulsion theory (VSEPR theory) enables us to predict the molecular structure, including approximate bond angles around a central atom, of a molecule from an examination of the number of bonds and lone electron pairs in its Lewis structure. When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass.įinding molar mass starts with units of grams per mole (g/mol). This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance. These relative weights computed from the chemical equation are sometimes called equation weights. This site explains how to find molar mass.įormula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. The reason is that the molar mass of the substance affects the conversion. To complete this calculation, you have to know what substance you are trying to convert.

In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.Ī common request on this site is to convert grams to moles.


 0 kommentar(er)
0 kommentar(er)
